Coccydynia, also known as tailbone pain, is perhaps the rare moment when we are actually aware of the presence of our vestigial tail. But let’s delve deeper. Imagine the convenience of living with a tail.* Both hands are occupied and you need to grasp something else? Just take a cue from the opossum with its prehensile tail adept at grabbing branches – it seems so effortless. If only it were not for an event that transpired around 25 million years ago, we might have been sipping our favourite drink while wielding cutlery in both hands. Instead, evolutionary pressure compelled us to abandon the arboreal lifestyle and adapt to a new style of locomotion. So, time to go back in time to unravel the genetic basis of tail-loss with researchers from New York University.
Without a time machine, teams led by Jef Boeke and Itai Yanai had to begin their detective work somewhere. Comparing sequences of genes predicted to be involved in tail-loss between two evolutionary closest animal lineages – one with tails and the other without, sounded like a reasonable starting point. Among these relatives were hominoids, encompassing humans and apes, thought to have lost their tails around 25 million years ago. This pivotal moment coincided with their divergence from their evolutionary siblings, ancient Old World monkeys, whose tailed family members include baboons, macaques, and mandrills. As genes involved in the loss of shortening of the tail were previously selected, scientists could narrow down their search to these specific genome parts of hominoids and Old World monkeys.
Genes are composed of coding segments known as exons and non-coding parts called introns. Despite being labelled as ‘non-coding’, these sequences are integral to a process called alternative splicing, where introns are removed from mRNA (messenger molecule carrying gene sequence). This process is incredibly powerful, as it can lead to rearrangements of various exons. Consequently, these mRNAs can be translated into different forms, known as isoforms, of a protein, that will eventually serve diverse functions within a cell.
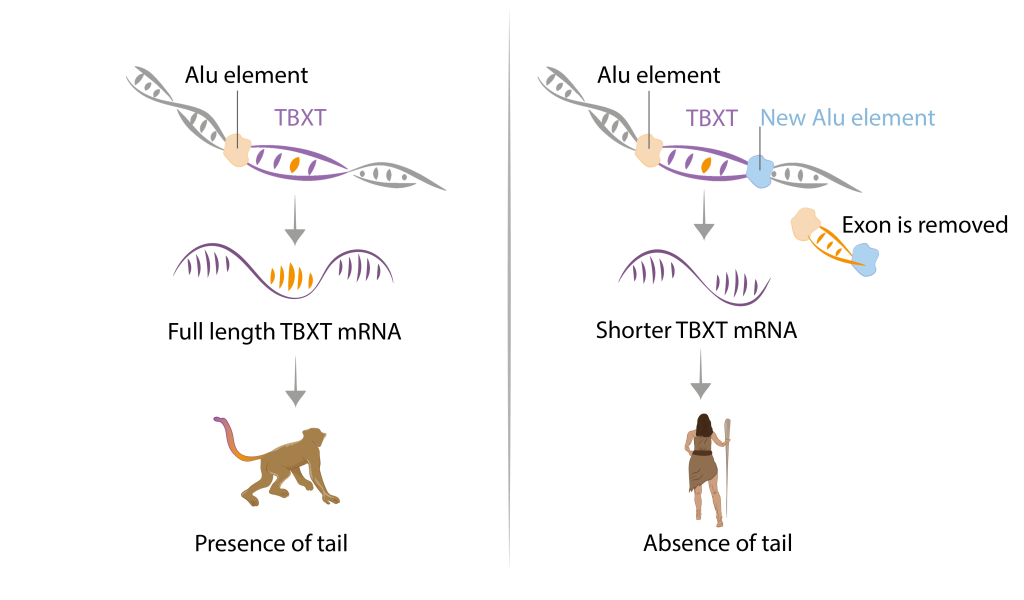
Upon closer investigation of gene sequences associated with the absence of a tail or its reduced forms, scientists made a significant discovery. They found an insertion within one of the introns of the hominoid TBXT gene. This inserted sequence to a type of Alu element, known as a transposable element or transposon. Transposons, also dubbed as ‘jumping genes’ or ‘tiny genome travellers’ are relics of an ancient virus infection. They populate up to 50% of our genome and their selfish goal is to replicate themselves. Hence, they jump around and insert in different regions of host genomes, sometimes disrupting genes in the process.** In this case, an insertion of the Alu element occurred around the same time when hominoids lost their tails. Moreover, it happened in a gene crucial for tail development. After a series of few elegant experiments later, researchers uncovered the mechanistic explanation of what transpired, leading to the birth of mice without tails.
The inserted Alu element interacts with the different Alu element that has been present before in the next intron. Together, they form a loop, causing an exon located between these two elements to be excised during alternative splicing. This results in the production of a shorter isoform of TBXT mRNA. However, merely generating a slightly shorter TBXT isoform is not sufficient to cause tail loss. Tail-less mice are only born in cases where there is either a lack of full-length TBXT or a significantly higher proportion of the shorter isoform.
As science is an endless journey of discovery, every result leads to new questions. This time was no different. Some of the mice without tails also exhibited defects in neural tube closure, a condition similar to the spina bifida in humans. While it may not be surprising given the importance of the TBXT gene during embryo development, it raises the question: Why would evolution deprive us of tails but leave us with the (rare) risk of developing serious health conditions?
* It is worth noting that animals also experience tail pain. Whether with or without a tail, life is not devoid of suffering.
** Rest easy knowing your cells have evolved mechanisms to curb the activity transposons.
References:
Xia, B., Zhang, W., Zhao, G., Zhang, X., Bai, J., Brosh, R., Wudzinska, A., Huang, E., Ashe, H., Ellis, G., et al. (2024). On the genetic basis of tail-loss evolution in humans and apes. Nature 626, 1042-1048. 10.1038/s41586-024-07095-8.