Blood is essential to maintain human life. Through blood circulation, nutrients absorbed in the digestive system and oxygen captured in the lungs are distributed to all organs.
However, life can be suddenly threatened when there is a reduction in oxygen transport due to severe blood loss. While blood transfusion is the most common alternative, shortage in blood stocks, compatibility issues, and the short life of blood cells are still challenges to overcome. In addition, in countries where infectious diseases, such as AIDS, affect many citizens, blood donations are rare.
Knowing this, scientists have been developing artificial products that are being tested to ensure oxygen delivery in emergency situations.
Oxygen transport is fulfilled by red blood cells or erythrocytes. These are cells present in very high amounts in the blood that contain a protein called hemoglobin. This protein has a center with iron, which captures oxygen molecules in the lungs and transports them to other tissues, where oxygen is released.
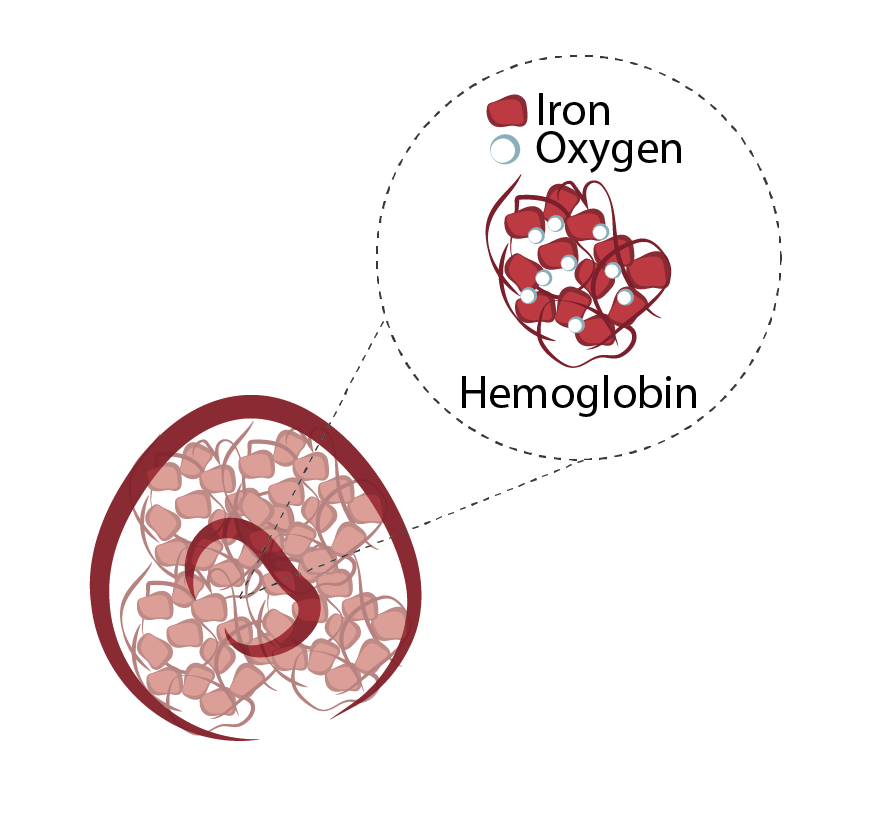
Figure 1- Illustration for red blood cells or erythrocytes. These cells without nucleus, contain inside a protein- Hemoglobin- that transports oxygen from lungs to the rest of the body. Hemoglobin contains iron, among other components. The iron is what confers these cells the color red and binds to oxygen molecules.
Artificial hemoglobin-based oxygen carriers (HBOCs) can potentially be injected into patients who have been through an accident in places where fresh blood is difficult to obtain, such as rural areas or war zones. Yet, the products tested so far contain only a hemoglobin-like molecule that could travel free through tissues and vessels and was not coated by a cell membrane, as usually happens in red blood cells. Although it can provide a fast solution to stabilize patients, these strategies often produce cardiac and vascular toxic effects, such as hypertension. For instance, given that hemoglobin is not surrounded by a membrane, oxygen changes occur very rapidly, which can cause cell oxidation in multiple organs (the same mechanism that makes apples and bananas brown after contact with air).
These toxic effects are also dependent on another molecule – Nitric Oxide. This molecule is usually produced by the blood vessels to allow their expansion and increase blood flow (for example, during exercise, this mechanism occurs in vessels near the muscles). After release, the levels of Nitric Oxide are controlled through its binding to hemoglobin. When using HBOCs, Nitric Oxide molecules are arrested by the free hemoglobin at a faster pace than when using red blood cells, causing the blood vessels to rapidly contract and causing hypertension. Therefore, a membrane that slows down these reactions could prevent the toxic effects of HBOCs.

Figure 2- Oxygen and nitric oxide (NO) dynamics in blood vessels. NO is released by cells that cover the blood vessels, expanding these and increasing blood flow. Red blood cells in the blood release the oxygen molecules and arrest NO, reducing the NO amount and causing the contraction of the blood vessels. When surrounded by a membrane (red blood cells or artificial red blood cells), these changes occur slowly. In common hemoglobin oxygen carriers (HBOCs), hemoglobin is free causing the rapid release of oxygen and arrest of NO, which can have toxic side effects, as oxidation and fast compression of the blood vessels and possible hypertension.
Artificial red blood cells could then offer an improved strategy for these patients. In this case, the hemoglobin protein is surrounded by a cell membrane composed by fat molecules, similar to natural red blood cells, preventing the toxic effects. Additionally, these artificial cells also have a much longer shelf life (~2 years) compared with bona fide red blood cells (~42 days) and are compatible with all blood types. Developed by a research team at the University of Maryland (UMD) School of Medicine, “ErythroMer” is made with human hemoglobin obtained from donated red blood cells that past their shelf life and is currently under preclinical testing. Although this product is currently dependent on hemoglobin from donated blood, the research team is developing a method to produce hemoglobin in the laboratory. This will increase “ErythroMer” availability.
In the future, artificial red blood cells can offer an unlimited blood substitute for patients in a critical state and may open the way for other artificial cell products.
For more information:
https://www.science.org/content/article/ultimate-blood-substitute-us-military-betting-46-million#


Pharmazee
Pharmazee Good post! We will be linking to this partspacelarly great post on our site. Keep up the great writing
Blue Tech
Blue Techker Great information shared.. really enjoyed reading this post thank you author for sharing this post .. appreciated