By Niklas Krausse
The current COVID-19 pandemic is still overshadowing many aspects of our lives. The fastest exit out of this situation seems to be the approval of a safe and effective vaccine. Scientists all over the world are eagerly looking for the holy grail – a protective vaccine – but how far have we come along?
Before we dive into ongoing clinical trials, let’s step back and reconsider the basic idea of vaccines again. A vaccine contains killed, weakened, or parts of a disease-causing pathogen (virus or bacteria). The vaccine educates our immune system to memorize the germ, without actually causing the disease. Once immunized against a disease through vaccination, the encounter with the real germ will evoke a much faster and stronger response of the immune system and prevent us from getting sick. That’s what makes vaccines so great, they do not treat a disease, but prevent the outbreak of the disease in the first place1.
Unfortunately, there is no approved vaccine available against coronaviruses yet. However, there is plenty of research under way, from pre-clinical studies (basic research performed before the drug is tested in patients) to clinical trials (drug testing in human patients). Let’s focus on some of the ongoing clinical trials (a more comprehensive list of ongoing studies can be found on the WHO website2).
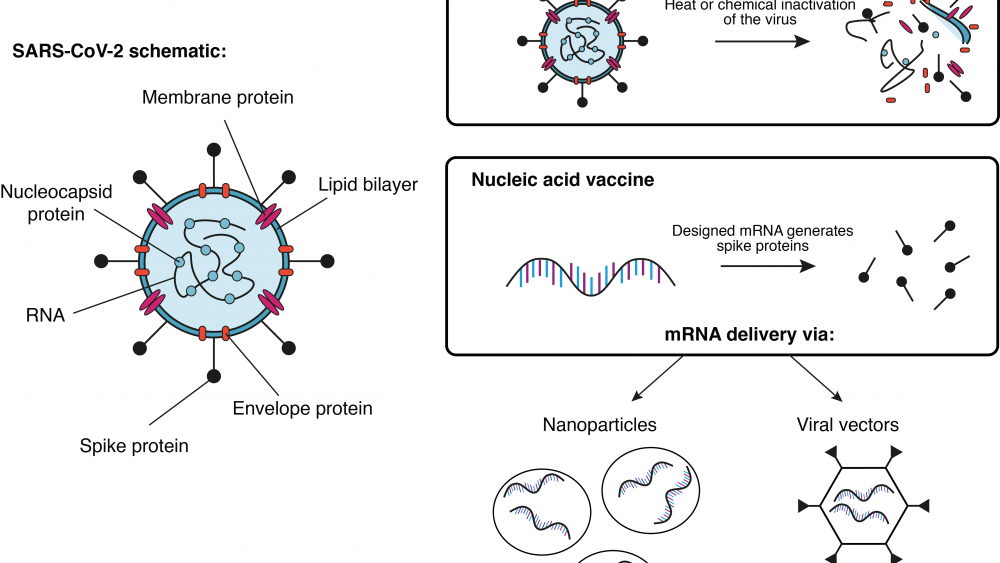
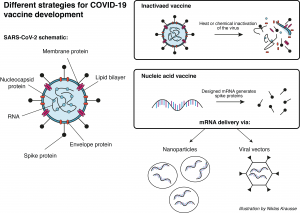
A group at Oxford University in the UK is working together with the pharmaceutical company AstraZeneca on a viral vector-based vaccine (see Infobox) called ChAdOx1-S. Here, researchers try to deliver the gene for the SARS-CoV-2 spike protein (see earlier blog post) to the patient’s cells. Once the cells produce the spike protein, the immune system generates a response against it. If sufficient immunity has been established, the patient is protected against an attack of the actual virus. So far, this vaccine platform has been successfully tested with the highly related middle east respiratory syndrome (MERS) virus in mice3 and monkeys4. Currently, a study from the group in Oxford using the vaccine platform to deliver the SARS-CoV-2 spike gene in monkeys is under review5. A similar vaccine approach has been developed by the Beijing Institute of Biotechnology and CanSino Biologics, both located in China. Their adenovirus type 5 vector has also entered clinical trials6.
Another approach has been pursued by Sinovac, the Wuhan Institute of Biological Products, and the Beijing Institute of Biological Products. All three have manufactured an inactivated vaccine for COVID-196 that has entered clinical trials by now. In this case, the actual virus is killed with heat or chemicals before it is used as a vaccine. Usually, these types of vaccines generate a strong protection against the intact pathogen. On the downside, the required safety precautions for the production and administration of such vaccines are naturally much higher.
The American biotech company Moderna, Inc. has launched clinical trials for their messenger RNA (mRNA) based vaccine against COVID-197. Here, mRNA molecules were artificially designed to contain only the building instructions for the SARS-CoV spike protein. The designed mRNA molecules were then encapsulated in nanoparticles for further delivery into the cells. Once in the host cells, the mRNA instructions are used to generate the viral protein, which then triggers the immune system to initiate a response. Similar technologies from other companies have entered the phase of clinical trials as well.
Among all the excitement and hope raised in finding an effective vaccine, it is important to keep a clear head. Vaccine development and testing takes time – and for good reason. The desired long-term effects of vaccines have to be carefully evaluated in terms of safety and efficient immune protection.
| Infobox: Different types of vaccines |
|---|
| Live attenuated vaccines: These vaccines contain a weakened version of the actual disease-causing germ. They usually provide strong and long-lasting immunity, but are not feasible for people with a weakened immune system, since they might cause the disease in these people. Inactivated vaccines: This type of vaccine contains whole germs that were killed before delivery to the patient. They are less efficient in generating immunity compared to live attenuated vaccines, but safer. Subunit vaccines: Subunit vaccines are another type of inactivated vaccines. Instead of the whole pathogen, subunit vaccines contain only a part of the germ (usually the part our immune cells recognize). Subunit vaccines are even safer than the previously mentioned vaccines, but usually elicit a weaker immune response. Nucleic acid vaccines: Nucleic acids such as DNA or RNA contain the building instructions for proteins. All cells have the machinery to translate these instructions into the actual proteins. Nucleic acid vaccines deliver the instructions for only some parts of the germ. Once built by the cell, the immune system recognizes the foreign proteins. However, the germs never get fully assembled, since only partial instructions were provided, thereby increasing safety. Viral vector vaccines: Here viruses themselves carry the vaccine. Harmless viruses are genetically modified to transport the vaccine to our cells (for example in the form of nucleic acids). One variation of this delivery platform are virus-like particles. In this case, harmless viruses carry proteins of the disease-causing pathogen on their surface. Tricked by this disguise, our immune system mounts a response against the displayed proteins. |